Exploring the Interface Between Two Nanoclusters: Insights from Computational Methods
DOI:
https://doi.org/10.37256/aecm.5120243746Keywords:
nanoclusters, HOMO-LUMO gap, aggregations, Interactions, dimerization, interface, orientations, self-assemblyAbstract
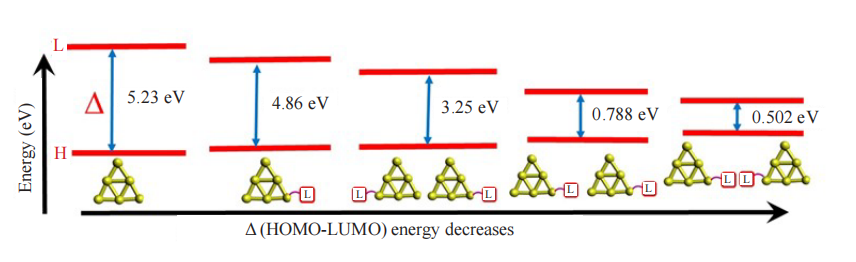
Aggregation of gold nanoclusters (GNCs) with desired properties requires detailed knowledge about the inter-cluster interface and its properties. The stability of [Au6]2 dimeric cluster configuration has been confirmed based on molecular dynamic simulation at 298 K temperature, 1 atm pressure, and water as solvent. The structural and electronic properties of series of monomeric and dimeric [Au6]2, [Au6H]2, [Au6CH3]2, [Au6C2H5]2, [Au6C5H9]2, [Au6C6H11]2, [Au6C6H5]2, [Au6C6H4CH3]2, [Au6C6H4CH3]2, [Au6C2H]2, [Au6C2CH3]2 and [Au6C2C6H5]2 clusters were studied using three different functionals in density functional theoretical methods by considering three different interfaces. The dimerization was found to alter the highest occupied molecular orbital-lowest unoccupied molecular orbital (HOMO-LUMO) gap depending upon the interface between the clusters. The computation predicts that the presence of ligand-ligand (ML-LM) and ligand-metal (ML-ML) interfaces in ligated cluster dimers were found to decrease the HOMO-LUMO gap while the metal-metal (LM-ML) interface leads to larger cluster formations. The change in electronic structures is found to be the reflection of symmetry in the eigenfunctions. The vertical plane of symmetry at the ML-LM interface leads to a smaller HOMO-LUMO gap as a result of degenerate orbitals between the monomeric units. The distortion from the reflection symmetry at the ML-ML interface removes the degeneracy by splitting the orbitals which increases the HOMO-LUMO gap in the ligated clusters. Among the studied ligated nanoclusters, experimentally realized [Au6C2C6H5]2 possesses a small HOMO-LUMO gap. All the interfaces are found to behave similarly in the presence of various uniform electric fields. The consequences of HOMO-LUMO gaps were observed in redox parameters, and absorption properties and have been explained using molecular orbital plots.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Ganga Periyasamy, Divya Maldepalli Govindachar

This work is licensed under a Creative Commons Attribution 4.0 International License.

