Band Gap Engineering of Spin-Coated α-Fe2O3 for Solar Water Splitting Utilizing a Facile Zinc Doping Route
DOI:
https://doi.org/10.37256/aecm.5220245492Keywords:
hematite, conduction band, valence band, photocatalyst, Zinc dopingAbstract
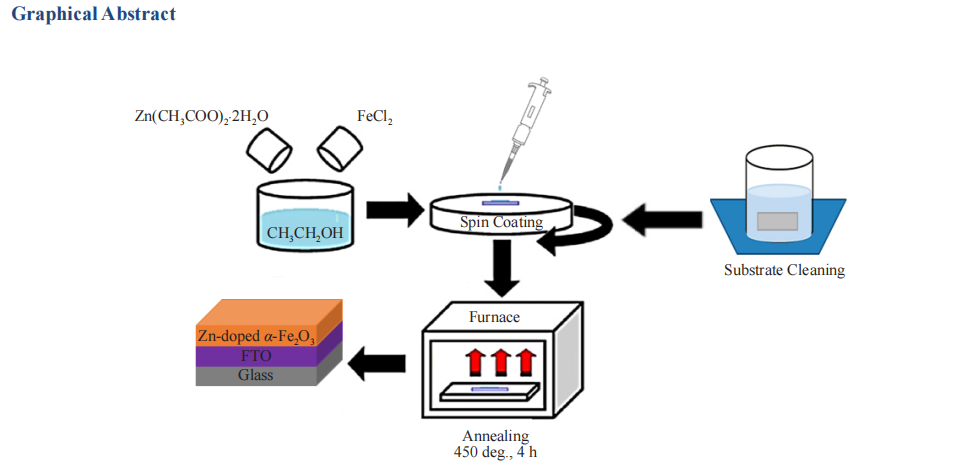
Iron oxide (hematite, α-Fe2O3) is an earth-abundant material having a favorable band gap for solar energy harvesting by producing Hydrogen (H2) by photoelectrochemical (PEC) water splitting. Although Fe2O3 has a valence band maximum (VBM) more positive than the water oxidation potential in the normalized hydrogen energy scale (NHE), it can not be used as a photocathode because of the more positive conduction band minima (CBM) than the reduction potential of the electrons required for the hydrogen evolution reaction (HER). This paper reports the band gap engineering of Fe2O3 by doping it with Zinc (Zn) through a facile way to modify its CBM and VBM to enable it to work as an ideal and efficient photocatalyst. One-pot synthesis of α-Fe2O3 doped with Zn was achieved by spin-coating a precursor solution prepared with FeCl2 and Zinc acetate (Zn(CH3COO)2.2H2O) on fluorine-doped tin oxide (FTO) or glass substrates. The films were then annealed in air at around 450 °C. The surface morphology and the crystal structure of the Fe2O3 were studied using SEM (Scanning Electron Microscope) and XRD (X-ray diffractometer). The UV-VIS spectrophotometry and spectroelectrochemistry (SEC) determined the band energies of α-Fe2O3 and Zn-doped α-Fe2O3. The band gap and CBM of pristine α-Fe2O3 were found to be -2.09 eV and -5.31 eV vs. EVAC, whereas the band gap and CBM of Zn-doped α-Fe2O3 were -1.96 eV and -4.3 eV vs. EVAC respectively. The outcome of the photoelectrochemical study shows that α-Fe2O3 doped with Zn can have a reduced band gap with more negative CBM than the H+/H2 reduction potential that efficiently ensures both oxygen and hydrogen evolution reaction.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Md. Ataur Raman, Md. Ferdouse Rahman, Abu Bakar Md. Ismail

This work is licensed under a Creative Commons Attribution 4.0 International License.

