Organotitanium Click Chemistry
DOI:
https://doi.org/10.37256/fce.4220232854Keywords:
organotitanium, azide, click chemistry, cycloaddition, insertion, bioactive, pharmaceuticalAbstract
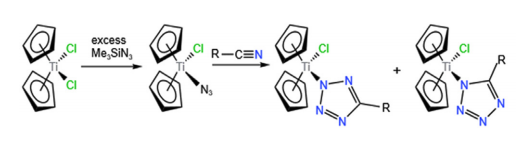
The click chemistry of titanium exemplified by the “green” [3 + 2] cycloaddition of the [Ti-N3] moiety with nitriles RCN to form a tetrazole ligand, is currently limited in scope, but the future of this open and wide field is bright, full of promising new synthetic approaches discussed herein, for pharmacological and bio orthogonal applications. This prediction is based on emerging click reaction possibilities from newly prepared titanium azides (complex 6), to existing titanium azides, including the commercially available Ti(N3)4, Cp2Ti(N3)2, (iPrO)2Ti(N3)2, and their multiple derivatives, participating in cycloaddition, exchange and other reaction types with alkynes, alkenes, nitriles, and related click synthons. Most of the organotitanium click reactions show nearly quantitative yields, as in the reaction of Ti(OiPr)4 with (CH3)3SiN3. Structural, mechanistic, stereo control and other effects on reactivity are briefly discussed. The main emphasis in this review article is on recently discovered organotitanium complexes like 2-phenylindole titanium dichloride, easily modified by insertion reactions of small molecules like CO2, SO2 or even RN3, serving as springboards of a new era in organotitanium click chemistry, by the original syntheses of non-toxic, potentially bioactive complexes.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Gregory G. Arzoumanidis

This work is licensed under a Creative Commons Attribution 4.0 International License.

