A New Fe2O3-Graphene-Hydrazine Sulphate Nanocomposite as Anode Material with Enhanced Electrocatalytic Activity in an H2O2 Fuel Cell
DOI:
https://doi.org/10.37256/sce.5120243756Keywords:
iron oxide, tafel polarization, electrocatalyst, H2O2, fuel cellAbstract
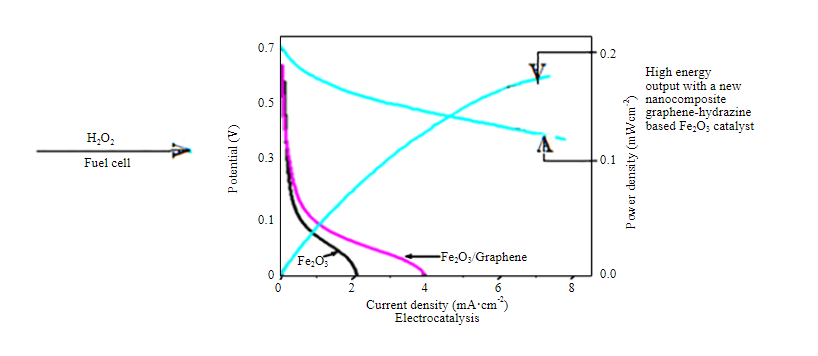
In the present study, synthesis of various phases of iron oxide was carried out by simple combustion method. The synthesized iron oxides and their composites with graphene and hydrazine sulphate are investigated as electrocatalytic materials for hydrogen peroxide (H2O2) fuel cells. The activity was seen to be dependent on the phase composition, surface features and electrical properties which was altered due to the use of the different amount of oxalic acid as one of the precursors during the synthesis. The catalysts which are more active for decomposition of hydrogen peroxide, showed lower electrocatalytic activity. A significantly improved electrocatalytic performance is obtained when a nanocomposite electrocatalyst is prepared using iron oxide, graphene and hydrazine sulphate.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Madhavi D. Shete, J. B. Fernandes

This work is licensed under a Creative Commons Attribution 4.0 International License.

