Binding and Specificity of Ovalbumin with Chitosan Derivatives Varying in Degree of Acetylation
DOI:
https://doi.org/10.37256/sce.5120243788Keywords:
chitosan, oligochitosan, ovalbumin, interactions, coacervation, nanoparticlesAbstract
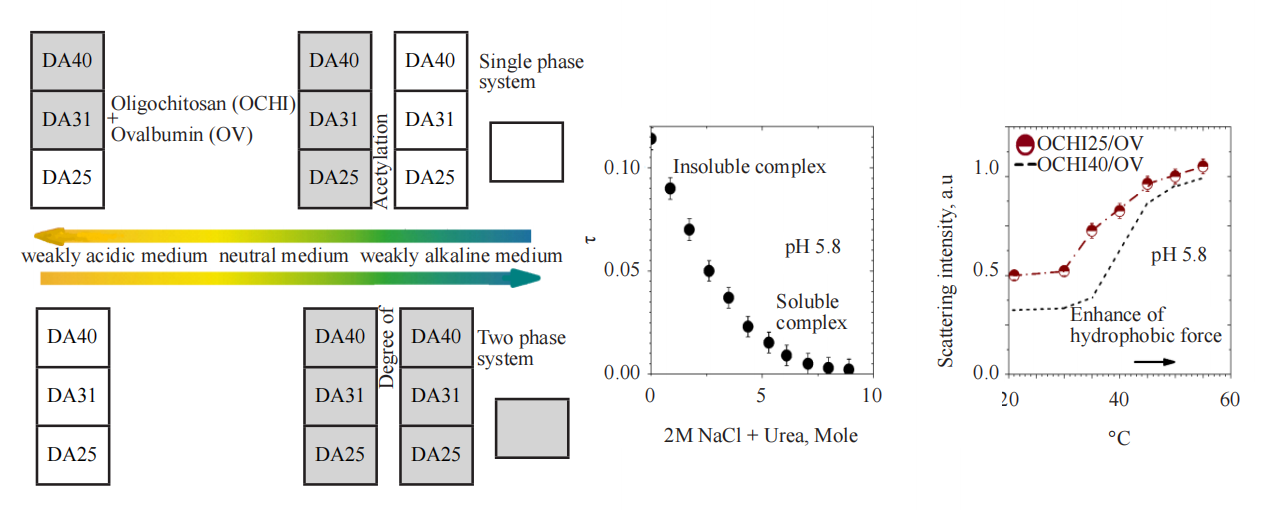
Here is an insight into the interaction and formation of insoluble complexes of ovalbumine (OV) with a series of specific chitosan derivatives (oligochitosan (OCHI)), varying in the degree of acetylation (DA) in acidic, neural and alkaline media. The forces involved in the complexation are determined and discussed. As shown, the interaction is partially electrostatic by nature with the “molar” composition in the insoluble OCHI-25/OV and OCHI-31/OV complexes of 1:2.8 and 1:2.1, respectively. The interaction is also partially hydrophobic and intensified at elevated temperatures. It is found that the binding of OV to OCHI and stability of the complexes depend on the way they are formed, and the affinity of OV to OCHI weakens with the increase in the DA. The complexes formed in a slightly acidic solution are limitedly soluble at a high solution ionic strength, and the aggregative state of the complexes depends on the weight fraction of OCHI in the complexes. The finding can be useful for understanding the complexation processes of chitosan with proteins and can be promising as a platform for the preparation of ovalbumin-based vaccines.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Irina L. Zhuravleva, Evgeniya A. Bezrodnykh, Victor N. Orlov, Boris B. Berezin, Vladimir E. Tikhonov, Yurij A. Antonov

This work is licensed under a Creative Commons Attribution 4.0 International License.

